US Access Board Requirements
Transfer surfaces shall be adjustable in height measured from the floor to the top of the
uncompressed transfer surface and shall provide the following:
- A low transfer position at 17 inches maximum
- A high transfer position at 25 inches minimum
- At least 4 additional transfer positions located between the low and high transfer positions
separated by 1 inch minimum
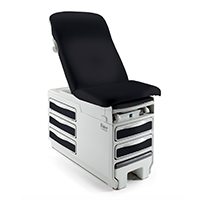
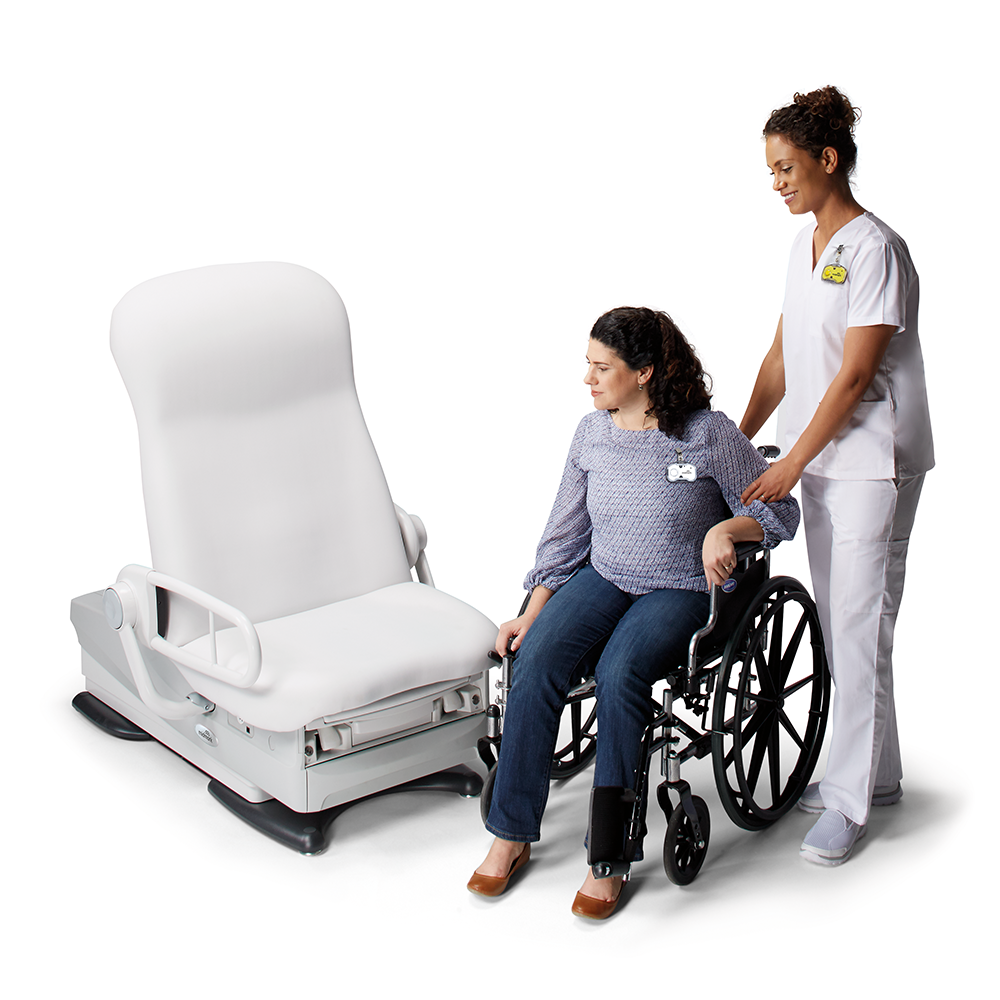
Midmark 626 Barrier-Free Examination Chair
The only chair on the market that can lower to a height of 15 ½ inches (uncompressed upholstery)
- High transfer position of 37 inches
- 21 ½ inches of height adjustability
US Access Board Requirements
The seated transfer surface shall be:
- 21 inches wide minimum
- 17 inches deep minimum
Midmark 626 Barrier-Free Examination Chair
- The seated transfer surface is 28 or 32 inches wide (depending on upholstery top)
- 19 ½ inches deep
US Access Board Requirements
The width of the base permitted within this clearance shall be 26 inches wide maximum at the edge
of the examination surface.
Midmark 626 Barrier-Free Examination Chair
- Base clearance of 23 ½ inches
- Compatible with patient lifts
US Access Board Requirements
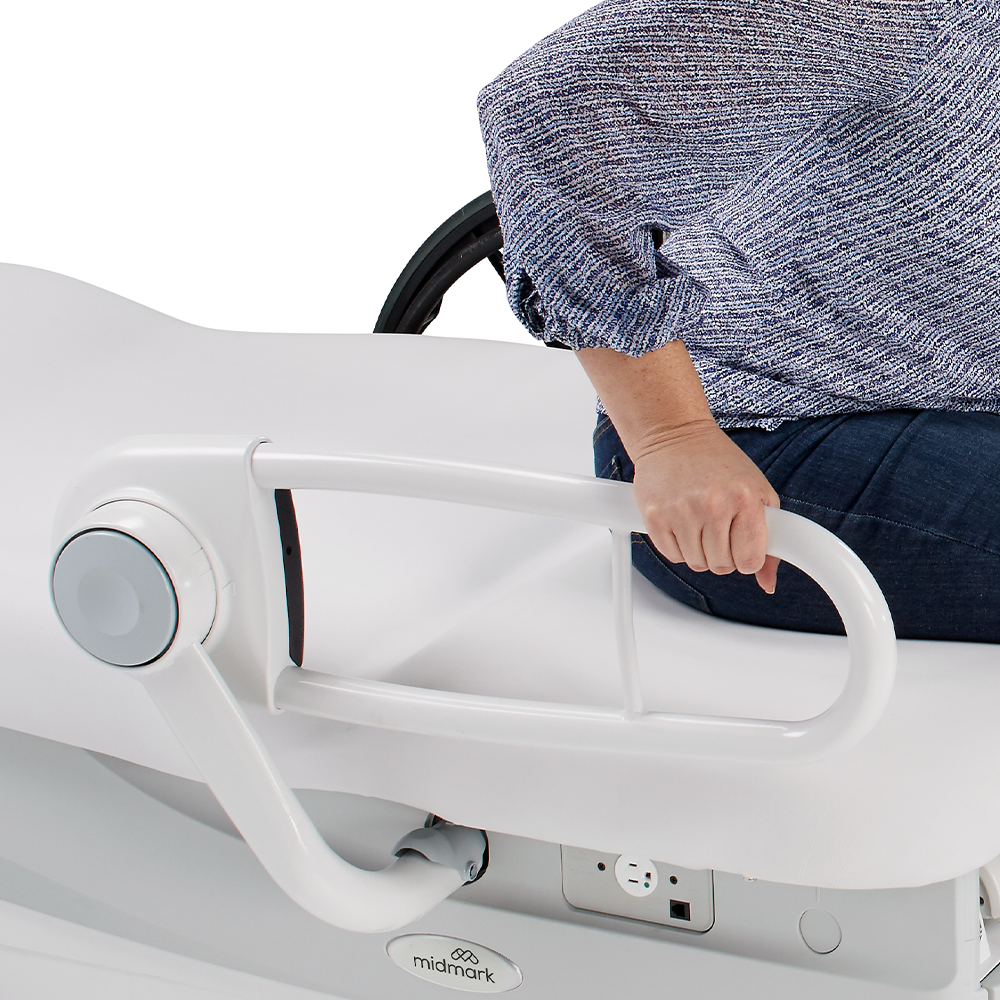
Transfer supports shall be/have:
- Located 1 ½ inches maximum measured horizontally from the plane defined by the nearest edge
of the transfer surface
- Capable of supporting transfer on each side of the transfer surface
- 15 inches long minimum
- 6 inches to 19 inches in height (higher than the top of the associated uncompressed transfer
surface) during use
- An outside diameter of 1 ¼ inches minimum and 2 inches maximum (circular cross section)
- A gripping surface continuous along their length and shall not be obstructed along their
tops or sides
Midmark Patient Support Rails and Patient Support Rails+
- Compliant to the new standards
- Provide patients with stability while entering, exiting or repositioning on the exam chair