US Access Board Requirements
The seated transfer surface shall be:
- 21 inches wide minimum
- 17 inches deep minimum
The ADA set the legal requirement. The US Access Board defined the standard. We're bringing it to you. Midmark is the first and only manufacturer in the market to have both a procedure and examination chair that complies with the US Access Board Standard.

Transfer surfaces shall be adjustable in height measured from the floor to the top of the uncompressed transfer surface and shall provide the following:
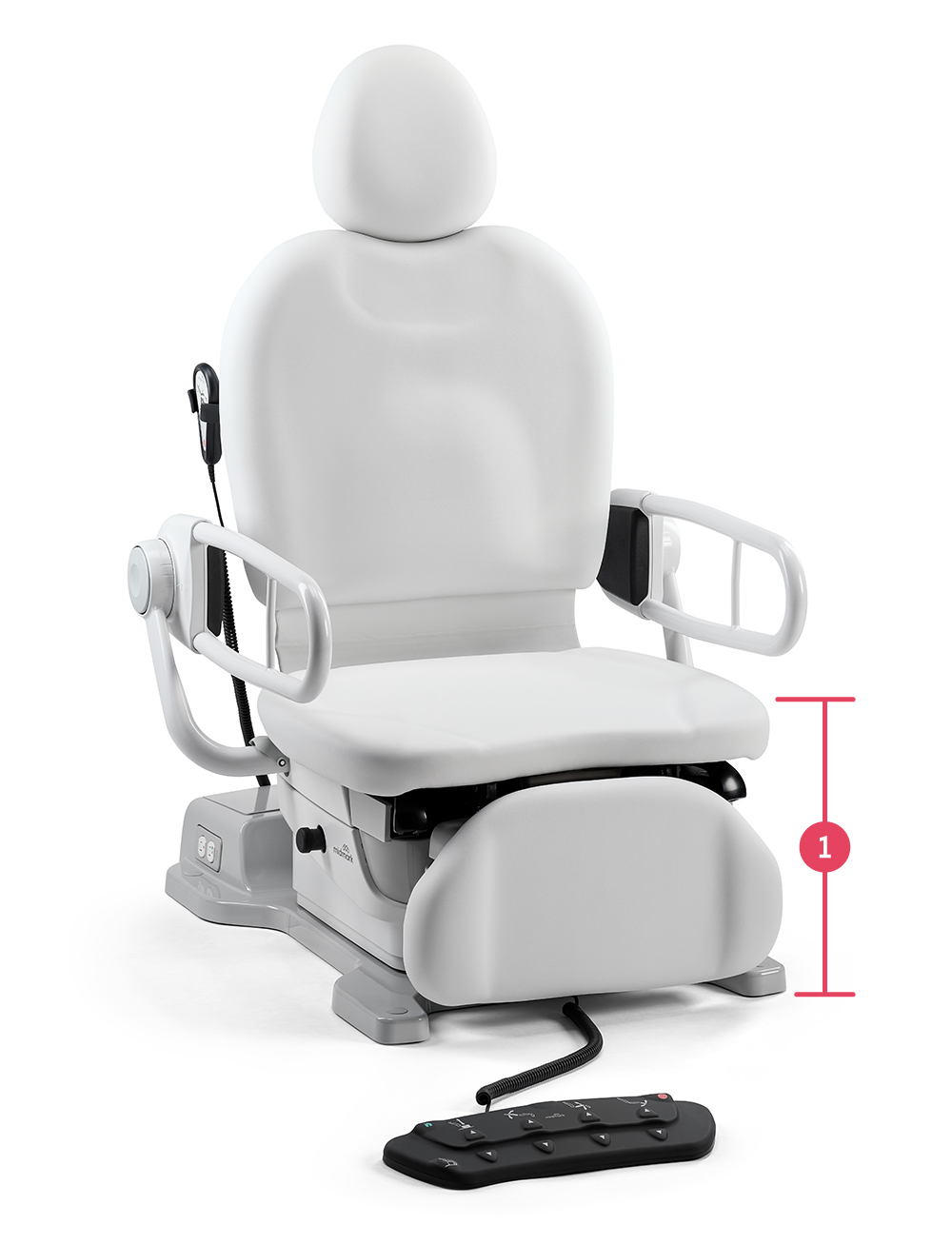
The first and only procedure chair in the market that can lower to a seat height of 17"
39" high seat height
22" of height adjustability

The first and only exam chair in the market that can lower to a seat height of 15 ½"
37" high seat height
21 ½" of height adjustability
The seated transfer surface shall be:
The width of the base permitted within this clearance shall be 26 inches wide maximum at the edge of the examination surface.
Transfer supports shall be/have:
Compliant to the standard, Midmark Patient Support Rails and Patient Support Rails+ assist patients in accessing the procedure and exam chair, and provide a stable gripping surface while repositioning on the chair.
Compliant to the standard. Midmark Articulating Knee Crutches support the patients thigh, knee and calf during lower body exams and procedures
Starting October 8, 2024, the Department of Justice is enforcing critical standards to improve accessibility in healthcare settings. These changes mean that medical diagnostic equipment in facilities across the country must now meet specific height and usability requirements to better serve people with disabilities. Discover what this means for healthcare providers, patients and caregivers alike by exploring the details below.
2 U.S. Census Bureau, American Community Survey, Disability Characteristics, https://data.census.gov/cedsci/table?t=Disability&tid=ACSST1Y2019.S1810
3 https://www.ncd.gov/assets/uploads/reports/ncd_medical_equipment_report_508.pdf
Want to dive deeper into accessible design? Explore these resources for expert insights, standards and practical tips to make your spaces more inclusive.
Take a moment to watch this brief video and share it with your colleagues to help raise awareness.
WATCH VIDEO
Watch this video for a quick overview of the design principles that can help you improve patient care and meet ADA requirements.
WATCH VIDEO
Learn more about the new Access Board Standard (MDE) and what makes the Midmark 626 and 631 compliant.
DOWNLOAD PDF
Explore ways to optimize your care environments for greater efficiency and improved patient experiences.
VISIT PAGE
Accessibility is required by the ADA, but state and Federal regulations can be complicated and cause confusion for health systems.
LEARN MORE
Invest in accessible, ADA-compliant equipment to see savings—calculate your IRC 44/179 tax credit and explore inclusive, efficient care solutions.
LEARN MORE
Curious about our products and services or have a general inquiry? We're here to answer your questions and provide assistance. Fill out the form and our team will reach out to you promptly.