-

 Anesthesia + MonitoringBrowse our industry-leading solutions and let us help you provide a better experience for all patients and providers
Anesthesia + MonitoringBrowse our industry-leading solutions and let us help you provide a better experience for all patients and providers -

 Comprehensive Dentistry SolutionsAdding dental services to your animal health practice promotes overall patient health—and it’s good for business.
Comprehensive Dentistry SolutionsAdding dental services to your animal health practice promotes overall patient health—and it’s good for business. -
 Animal Housing
Animal Housing
Building your dream clinic can be both exciting and scary. But you don’t have to do it alone. Let us help you design a space perfectly suited to you and your team.
Make Your Dream Clinic A Reality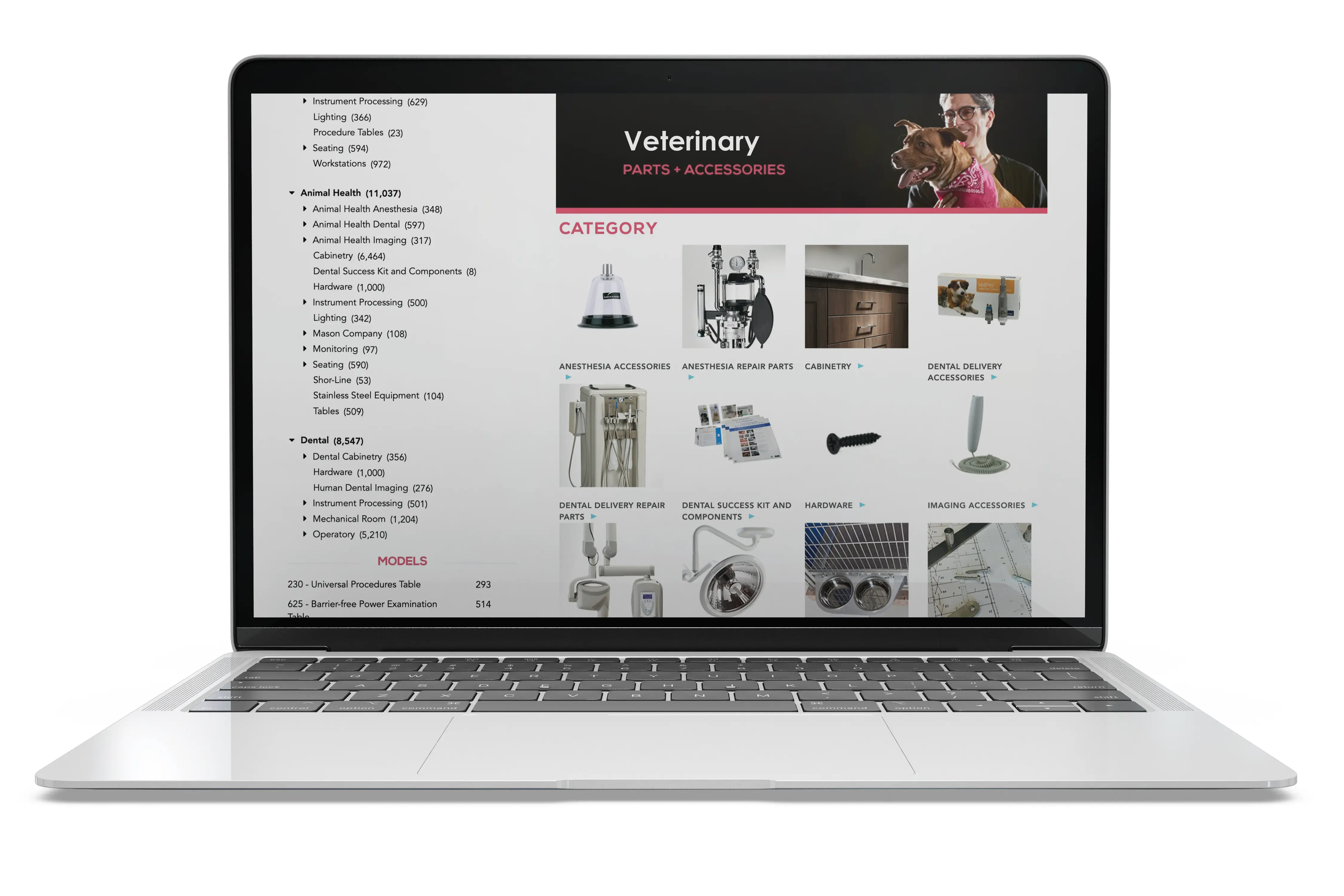
Explore parts and accessories for your veterinary products.
Shop Now2025 Dental Imaging Training + Install Program
2024-2025 Sterilizer Maintenance Kit Promotion
-
BlogLet’s talk about better care. Join the discussion today.
-
Product CatalogsDesigning the best animal care experience isn’t always easy. We can help.
-
Product LiteratureSee how we’re helping our customers design better, more efficient care.
-
VideosWe’re committed to innovation and are grounded in learning – see what we’ve uncovered.
Education
Services
Support
-
 Product Manuals (Technical Library)
Product Manuals (Technical Library) -

 Shop Parts, Software + ServicesVisit The Online Parts Store for 24/7 ordering.
Shop Parts, Software + ServicesVisit The Online Parts Store for 24/7 ordering. -
 Animal Health Technical SupportLet our dedicated technical service team help you find parts, documentation and more.
Animal Health Technical SupportLet our dedicated technical service team help you find parts, documentation and more. -

 Customer ExperienceOur friendly support team is here to assist you every step of the way.
Customer ExperienceOur friendly support team is here to assist you every step of the way.